EEK Global Presence
Established in 1992, EEK(En'ErKang) has been serving domestic pharmaceutical prescription market for 3 decades. Headquartered in Zhejiang, China, EEK also has branches in Qingdao and Hong Kong.
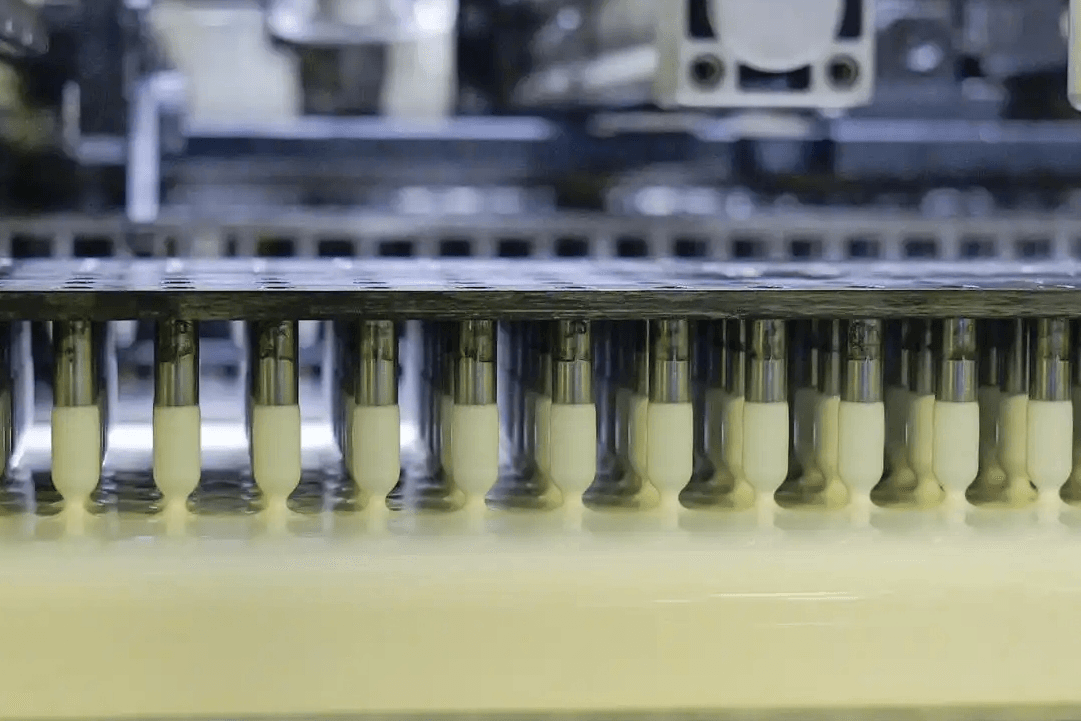
With 14 production lines, EEK is able to produce over 7 billion pieces of quality capsules annually. Thanks to a premier set of technologies, expertise and infrastructure, we’d meet the evolving challenges faced by biopharmaceutical customers.

EEK Product and Services
Capsules are available in the entire size range (000, 00, 00el, 0el, 0, 1, 2, 3, 4 and the very special tiny size 5) and in a wide range of colors and with linear and circular two & four printing possibilities.
Unique printing from EEK serves as an excellent anti-counterfeit measure to safeguard you brand and business interests.We also have a team of competent staff having hands on experience on highly sophisticated automatic capsule filling in capsule manufacturing.
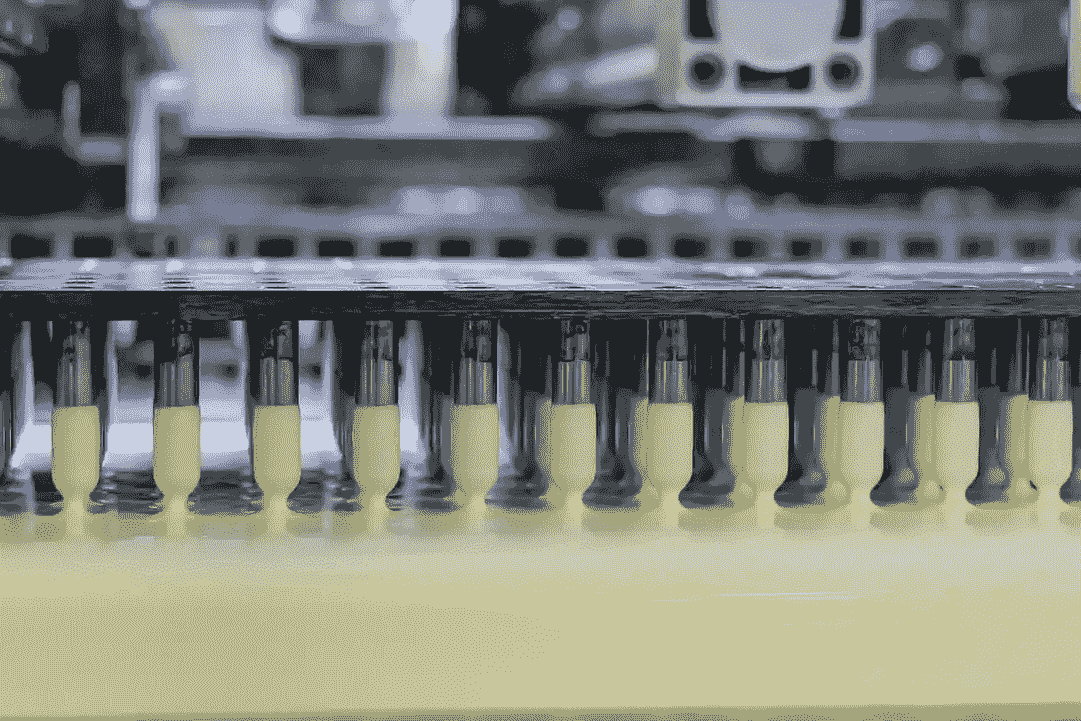
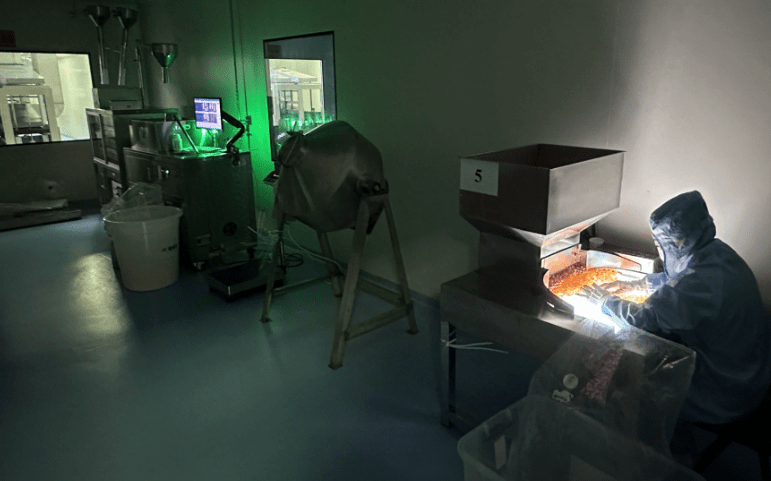
EEK State-of-the-art Manufacturing Facility
Quality Assurance plays an integral part in monitoring all critical process parameters like, temperature and humidity, measurable parameters like, weight, length, diameter and thickness.
A systematic approach that eliminates error in every stage of production to meet customer requirement. The last stage of the journey begins with the initial packaging of defect free capsules in non-static polyethylene bags to hold sufficient quantity of capsules based on capsule size.

Quality, Variety and Consistency
Quality Control starts with usage of the best indigenous pharmaceutical grade HPMC–the natural raw material required for manufacturing. A thorough inspection and continuous monitoring of critical process and measurable parameters present Zero Defect products.
Regular and planned hands-on workshop addressing the training needs enable us to maintain consistency. The quality policy and objectives are reviewed in every management review meeting for continuing suitability.

EEK Headquarter
588 Fenghe Road
Pujiang
Zhejiang, China
EEK Qingdao
45 Beijing road
Qingdao
Shandong, China
EEK Hong Kong
6703 The Center
99 Queen's Road
Central, HK
© 2025 Copyright © EEK. All rights reserved. Privacy & HSE policies.